Inhibiting Sphingosine-1-Phosphate Receptor 3 (S1PR3) Mitigates Pulmonary Fibrosis
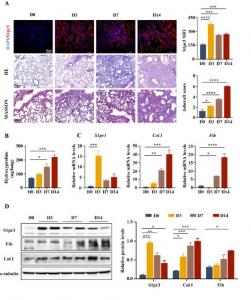
(A) Representative images of S1pr3 immunofluorescence staining (upper panel, scale bar = 50 μm), HE staining (middle panel, scale bar = 100 μm), and Masson's trichrome staining (lower panel, scale bar = 25 μm) of lung tissues after BLM induction at differ
S1pr3 knockdown ameliorates bleomycin-induced pulmonary fibrosis by reducing macrophage M2 polarization
CHINA, March 28, 2025 /EINPresswire.com/ -- Pulmonary fibrosis (PF) involves the excessive accumulation of extracellular matrix, resulting in the loss of lung architecture and a decline of lung function, ultimately culminating in respiratory failure and death. Despite the availability of new effective drugs, the incidence and mortality of PF continue to rise, while its pathogenesis remains poorly understood. An early inflammatory event triggers the onset of an abnormal reparative process, leading to fibrosis. Controlling the M2 macrophage population represents a promising therapeutic strategy; however, identifying the mechanisms regulating their polarization is crucial.
In a recent study published in the Genes & Diseases journal, researchers at Children's Hospital of Chongqing Medical University and Children's Hospital of Shanghai Jiaotong University Medical School showed that knockdown or inhibition of sphingosine-1-phosphate receptor 3 (S1pr3), a receptor for the lipid signaling molecule sphingosine-1-phosphate, ameliorates bleomycin (BLM)-induced PF in mice marked by a decrease in M2 macrophage infiltration into the lung resulting from the attenuation of M2 macrophage polarization via repression of the PI3K/Akt-Stat3 signaling pathway.
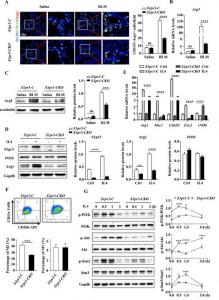
The authors showed that i) bleomycin upregulates the expression of S1pr3 to the highest levels during the early stages of fibrosis, with a concomitant increase in the expression of the fibrotic markers collagen I and fibronectin, ii) S1pr3 expression was significantly higher in macrophages in mouse PF tissue, and iii) S1pr3 overexpression promotes M2 macrophage polarization in BLM-induced PF.
Further studies with a myeloid-specific S1pr3 knockout mouse model (the LysM-Cre+/S1pr3flox/flox mice, defined as S1pr3-CKO mice) and their littermates (the LysM-Cre−/S1pr3flox/flox mice, defined as S1pr3-C mice) as controls showed that i) the specific deletion of S1pr3 in macrophages ameliorates BLM-induced lung damage and fibrosis in mice, and ii) S1pr3 regulates the progression of PF by affecting the infiltration of M2 macrophages into the lung.
Additional experiments with bone marrow-derived macrophages (BMDMs) generated from S1pr3-C and S1pr3-CKO mice and subjected to IL-4 stimulation indicated that S1pr3 deficiency attenuates IL-4-induced macrophage M2 polarization. IL-4 stimulation significantly increases p-PI3K, p-Akt, and p-Stat3 levels in S1pr3-C BMDMs compared to S1pr3-CKO BMDMs, suggesting that S1pr3 affects macrophage M2 polarization by regulating the PI3K/Akt-Stat3 signaling pathway. Furthermore, administering the S1pr3 inhibitors CAY10444 and TY52156 in the early inflammatory phase of BLM-induced lung injury ameliorated fibrosis by reducing M2 macrophage polarization, suggesting that S1pr3-specific inhibitors represent a viable intervention against PF.
In conclusion, this study shows that S1pr3 contributes to PF by modulating macrophage M2 polarization through the PI3K/Akt-Stat3 signaling pathway. In addition, S1pr3 inhibitors were effective against PF in mice, hinting that these molecules represent a potential therapeutic strategy in humans.
Reference
Title of the original paper: Inhibition of sphingosine 1-phosphate receptor 3 ameliorates bleomycin-induced pulmonary fibrosis by suppressing macrophage M2 polarization.
Journal: Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI: https://doi.org/10.1016/j.gendis.2024.101244
Funding Information:
General Basic Research Project from the Ministry of Education Key Laboratory of Child Development and Disorders (China) (No. GBRP-202115)
The Chongqing Science and Technology Bureau Major Project (China) (No. cstc2020jcyj-msxmX0782).
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Education, Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release