Apoptotic Vesicles Loaded Fibrous Scaffolds Promote Wound Healing
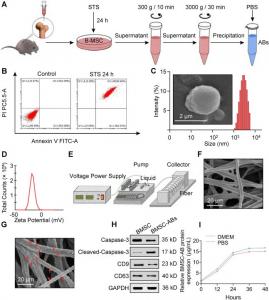
(A) Acquisition of BMSC-ABs. (B) Identification of apoptotic cells by flow cytometry. (C) Morphological images of ABs. Left: scanning electron microscopy (SEM) images of ABs (scale bar, 2 μm); right: particle size of ABs measured by dynamic light scatteri
PCL fiber scaffolds loaded with BMSC-derived apoptotic vesicles regulate macrophage polarization via miR-21a-5p to promote wound healing
CHINA, March 6, 2025 /EINPresswire.com/ -- A recent study showed that paracrine delivery of miR-21a-5p by bone marrow mesenchymal stem cells-derived apoptotic bodies (BMSC-derived AB)-loaded polycaprolactone (PCL) scaffolds promote wound healing by regulating macrophage polarization to M2 phenotype.
Macrophages are critical players mediating wound healing, and the microenvironment of the wound influences macrophage polarization to either the M1 pro-inflammatory phenotype in the early stages or the M2 pro-healing phenotype in the later stages. Dysfunction of the macrophage polarization M2 phenotype, lower macrophage numbers, and reduced anti-inflammatory and angiogenic capacity are causatives of long-term non-healing.
Mesenchymal stem cells (MSCs) aid tissue repair and regeneration by delivering endogenous regulatory factors via paracrine extracellular vesicles. Previous studies have shown that BMSC-derived ABs induce macrophage polarization to the M2 phenotype, which mitigates inflammation and enhances the migration and proliferation of fibroblasts.
In this study, published in the Genes and Diseases journal, researchers at Chongqing Medical University and Sichuan Provincial Orthopedic Hospital investigate the wound healing efficacy and macrophage polarization potential of electrospun PCL scaffolds loaded with BMSC-ABs, in vitro and in vivo.
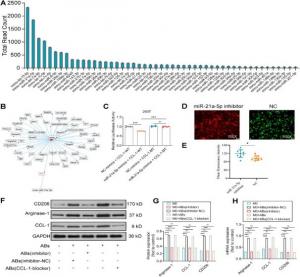
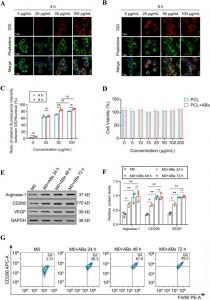
The results showed that the BMSC-AB loaded PCL scaffolds could prevent or reduce delayed wound healing by reprogramming the macrophages into the M2 phenotype and reducing inflammatory infiltration, thereby potentiating anti-inflammatory and angiogenic effects. Furthermore, the paracrine delivery of miR-21a-5p through the slow-release of BMSC-AB by the PCL scaffolds reprograms macrophages to the M2 phenotype by regulating the expression of the CCL-1 gene, promotes fibroblast migration, and initiates angiogenesis by secreting anti-inflammatory cytokines and the angiogenesis-related factors, VEGF and vWF.
In conclusion, the BMSC-AB-loaded PCL scaffolds, through the targeted and sustained delivery of miR-21a-5p, drive macrophage polarization to the M2 phenotype, which exerts a dual effect by regulating inflammation and angiogenesis, thereby synergistically promoting wound healing.
Reference
Title of the original paper: Fibrous scaffolds loaded with BMSC-derived apoptotic vesicles promote wound healing by inducing macrophage polarization.
Journal: Genes & Diseases
Genes & Diseases is a journal for molecular and translational medicine. The journal primarily focuses on publishing investigations on the molecular bases and experimental therapeutics of human diseases. Publication formats include full length research article, review article, short communication, correspondence, perspectives, commentary, views on news, and research watch.
DOI: https://doi.org/10.1016/j.gendis.2024.101388
Funding Information:
National Natural Science Foundation of China (No. 82072443, 82372425)
The Chongqing Outstanding Project of Overseas Chinese Entrepreneurship and Innovation Support Program (China) (No. CX2022032)
The Articular Cartilage Tissue Engineering and Regenerative Medicine Team, Chongqing Medical University (No. W0080)
The General Project of Natural Science Foundation of Chongqing, China (No. CSTB2023NSCQ-MSX0166)
The “Tomorrow Cup” Teacher‒Student Cocreation Teaching and Research Innovation Project of International Medical College of Chongqing Medical University (No. KY20220204)
The Chengdu Medical Research Project (Sichuan, China) (No. 2023191)
The China Postdoctoral Science Foundation (No. 2022M710557)
The Natural Science Foundation of Chongqing, China (No. CSTB2023NSCQ-BHX0011)
The Young Excellent Science and Technology Talent Project of the First Affiliated Hospital of Chongqing Medical University (No. ZYRC2022-05)
# # # # # #
Genes & Diseases publishes rigorously peer-reviewed and high quality original articles and authoritative reviews that focus on the molecular bases of human diseases. Emphasis is placed on hypothesis-driven, mechanistic studies relevant to pathogenesis and/or experimental therapeutics of human diseases. The journal has worldwide authorship, and a broad scope in basic and translational biomedical research of molecular biology, molecular genetics, and cell biology, including but not limited to cell proliferation and apoptosis, signal transduction, stem cell biology, developmental biology, gene regulation and epigenetics, cancer biology, immunity and infection, neuroscience, disease-specific animal models, gene and cell-based therapies, and regenerative medicine.
Scopus CiteScore: 7.3 | Impact Factor: 6.9
# # # # # #
More information: https://www.keaipublishing.com/en/journals/genes-and-diseases/
Editorial Board: https://www.keaipublishing.com/en/journals/genes-and-diseases/editorial-board/
All issues and articles in press are available online in ScienceDirect (https://www.sciencedirect.com/journal/genes-and-diseases).
Submissions to Genes & Disease may be made using Editorial Manager (https://www.editorialmanager.com/gendis/default.aspx ).
Print ISSN: 2352-4820
eISSN: 2352-3042
CN: 50-1221/R
Contact Us: editor@genesndiseases.com
X (formerly Twitter): @GenesNDiseases (https://x.com/GenesNDiseases)
Genes & Diseases Editorial Office
Genes & Diseases
+86 23 6571 4691
email us here
Visit us on social media:
Facebook
X
LinkedIn
Instagram
YouTube
Other
Distribution channels: Healthcare & Pharmaceuticals Industry, Science
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release