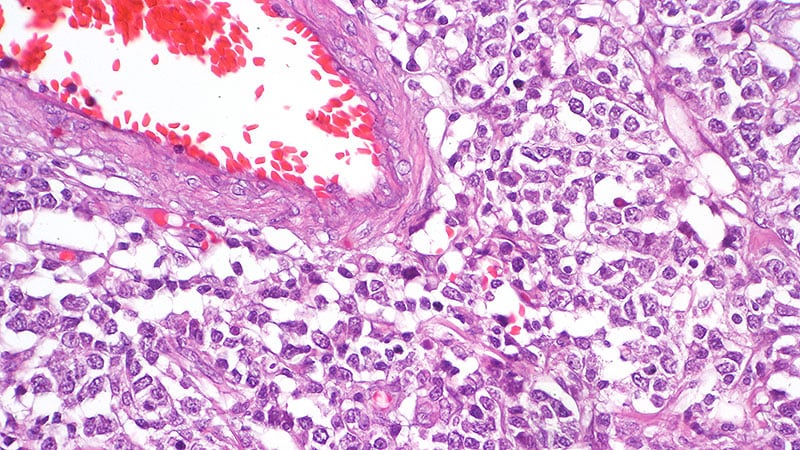
Phase 3 trial initiated for Ifinatamab Deruxtecan in Pretreated Metastatic Castration-Resistant Prostate Cancer: Merck, ...
Basking Ridge: Merck and Daiichi Sankyo have announced that the first patient has been dosed in the IDeate-Prostate01 phase 3 trial evaluating the efficacy and safety of investigational ifinatamab deruxtecan (I-DXd) versus docetaxel in patients with …