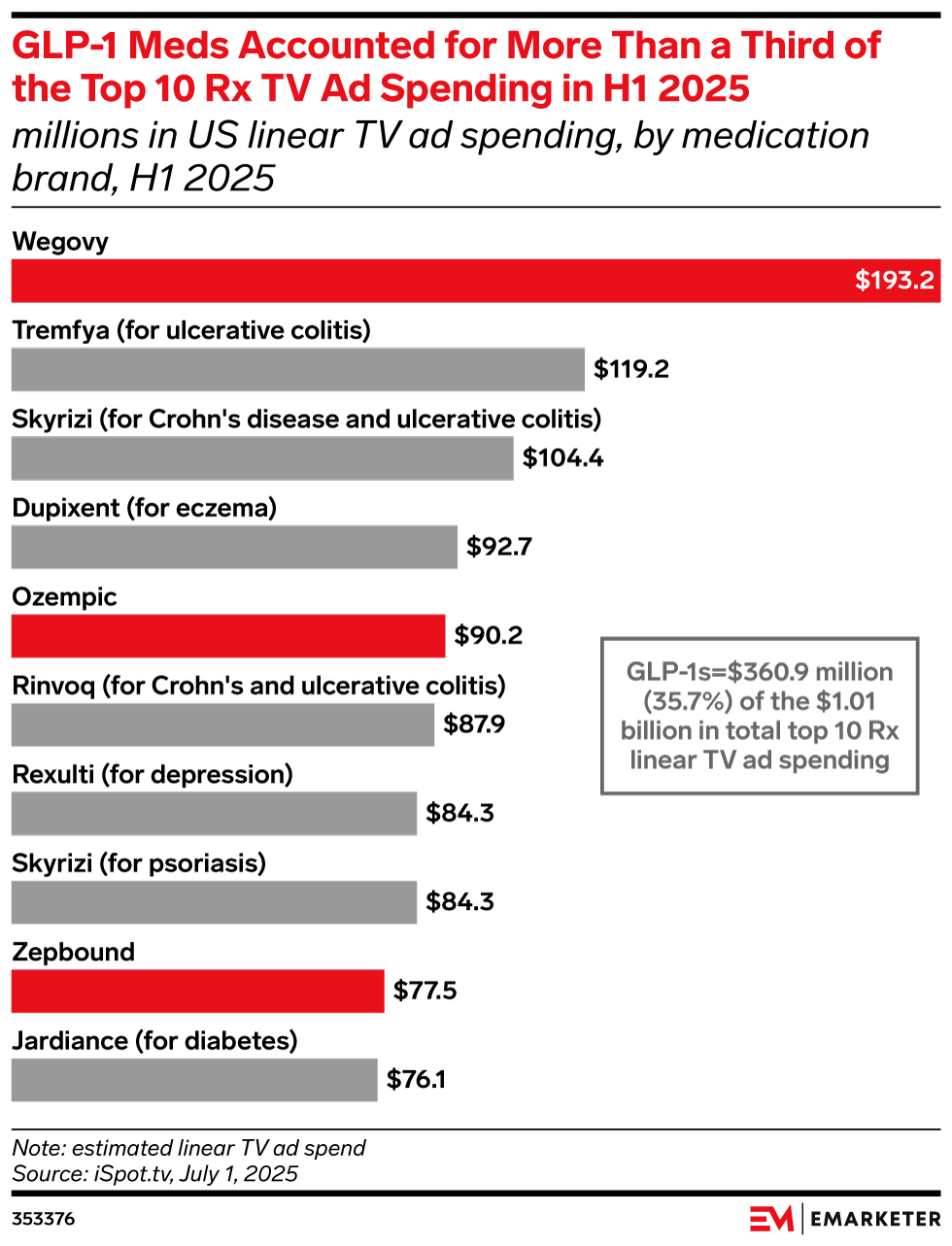
I'd Like A Prescription Against Prescription Meds TV Ads
Whether you lean right or left, everyone can probably agree that Oh-Oh-Oh-Ozempic is advertising too much. Our current Health and Human Services Secretary, Robert F. Kennedy Jr., has long criticized the practice of allowing pharmaceutical companies to …



:max_bytes(150000):strip_icc()/VWH-GettyImages-1416464231-d63941c266e04e3fab6b83498e64a033.jpg)