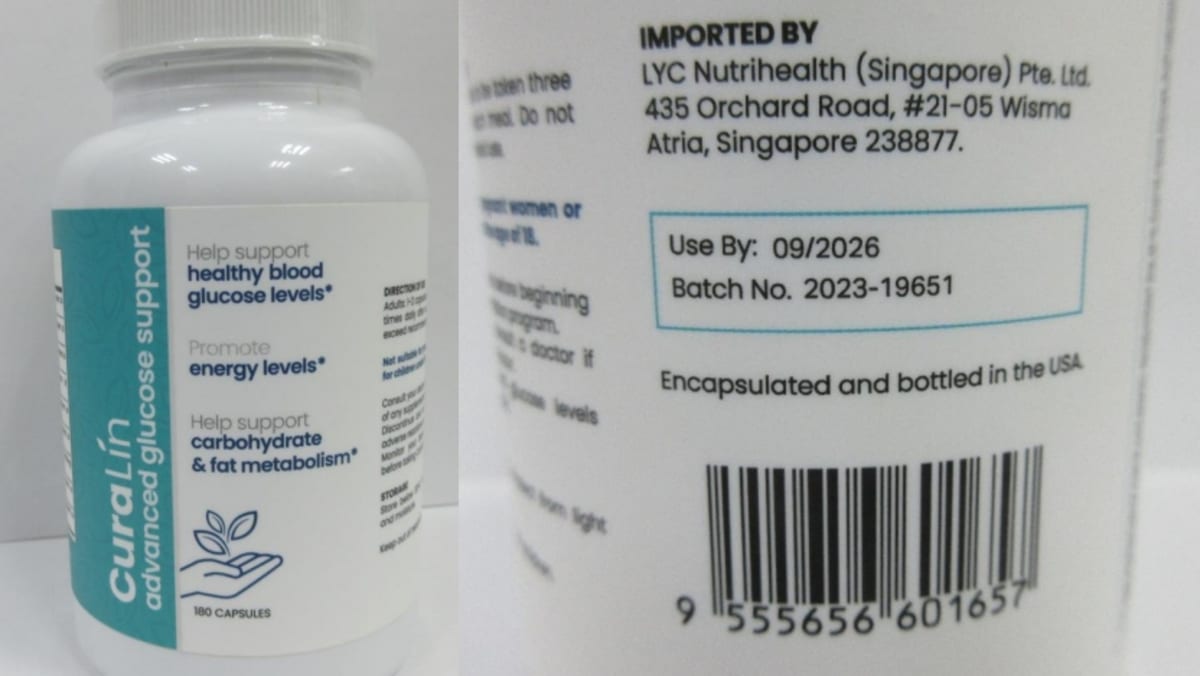
Lupin wins USFDA okay for HIV-1 drug Raltegravir
Mumbai: Global pharma major Lupin Limited has announced that the United States Food and Drug Administration (USFDA) has approved the Company's Abbreviated New Drug Application for Raltegravir Tablets USP, 600 mg. Raltegravir Tablets are bioequivalent …