FDA Approves Avutometinib Plus Defactinib for KRAS-Mutated Recurrent Low-Grade Serous Ovarian Cancer
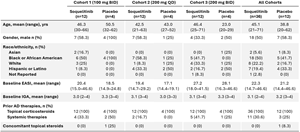
The FDA has granted accelerated approval to avutometinib plus defactinib (Avmapki Fakzynja Co-pack) for adult patients with KRAS-mutated recurrent low-grade serous ovarian cancer (LGSOC) who have previously received systemic therapy. The regulatory …






:max_bytes(150000):strip_icc():focal(1023x0:1025x2)/baby-dies-whooping-cough0_05052025-6a41be2a10a0435cb401b64330cfcfc0.jpg)