FDA Grants Accelerated Approval to Avutometinib/Defactinib in KRAS+ LGSOC
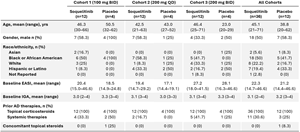
The News The FDA has approved avutometinib in combination with defactinib (Avmapki Fakzynja Co-pack) as a treatment for adult patients with recurrent, KRAS-mutated, low-grade serous ovarian cancer (LGSOC), according to a press release from the agency.1 …