
Sarcopenia Clinical Trial Pipeline Appears Robust With 18+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The sarcopenia market remains largely untapped, with significant unmet medical needs and no approved drug therapies in major markets. As global demographics shift toward older populations, pharmaceutical interest is rising, particularly around muscle-enhancing agents and anti-inflammatory pathways. Regulatory bodies like the FDA and EMA are beginning to recognize sarcopenia as a distinct clinical condition, which may streamline future drug approvals. Meanwhile, the convergence of lifestyle interventions, personalized medicine, and digital health solutions presents a multi-modal approach to disease management. Early movers in this space have the potential to shape clinical guidelines and capture long-term market leadership.
/EIN News/ -- New York, USA, April 24, 2025 (GLOBE NEWSWIRE) -- Sarcopenia Clinical Trial Pipeline Appears Robust With 18+ Key Pharma Companies Actively Working in the Therapeutics Segment | DelveInsight
The sarcopenia market remains largely untapped, with significant unmet medical needs and no approved drug therapies in major markets. As global demographics shift toward older populations, pharmaceutical interest is rising, particularly around muscle-enhancing agents and anti-inflammatory pathways. Regulatory bodies like the FDA and EMA are beginning to recognize sarcopenia as a distinct clinical condition, which may streamline future drug approvals. Meanwhile, the convergence of lifestyle interventions, personalized medicine, and digital health solutions presents a multi-modal approach to disease management. Early movers in this space have the potential to shape clinical guidelines and capture long-term market leadership.
DelveInsight’s 'Sarcopenia Pipeline Insight 2025' report provides comprehensive global coverage of pipeline sarcopenia therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the sarcopenia pipeline domain.
Key Takeaways from the Sarcopenia Pipeline Report
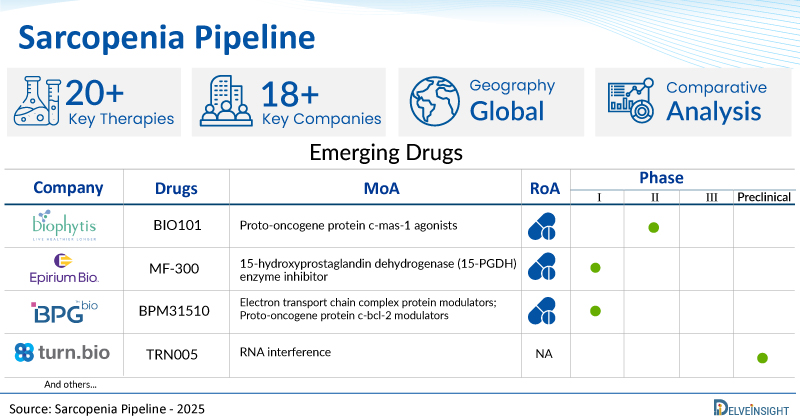
- DelveInsight’s sarcopenia pipeline report depicts a robust space with 18+ active players working to develop 20+ pipeline sarcopenia drugs.
- Key sarcopenia companies such as Biophytis, TNF Pharmaceuticals, Epirium Bio, Turn Biotechnologies, Oncocross, EUSOL Biotech, BPGbio, Inc., Lipocine, and others are evaluating new sarcopenia drugs to improve the treatment landscape.
- Promising pipeline sarcopenia therapies such as BIO101, MYMD-1, MF-300, TRN005, ES-1602, OC514, BPM31510, and others are in different phases of sarcopenia clinical trials.
- In April 2025, TNF Pharmaceuticals, Inc. announced that a platform presentation of the abstract titled “Isomyosamine for the Treatment of Sarcopenia in Older Adults” was delivered by Mitchell Glass, M.D., President and Chief Medical Officer of TNF, at the British Geriatrics Society (BGS) Spring Meeting 2025, held April 9–11 in Belfast, Ireland, and online.
- In December 2024, Epirium Bio, Inc. (Epirium) announced that the US Food and Drug Administration (FDA) has cleared the Investigational New Drug (IND) application for MF-300, a first-in-class orally administered, 15-hydroxyprostaglandin dehydrogenase (15-PGDH) enzyme inhibitor in development for the treatment of sarcopenia, or age-induced muscle weakness.
- In December 2024, Lipocine announced that the US Food and Drug Administration (FDA) had granted Fast Track Designation to LPCN1148 as a treatment for sarcopenia in patients with decompensated cirrhosis.
- In October 2024, Rejuvenate Biomed, the University of Leicester, the National Institute for Health and Care Research (NIHR) Leicester Biomedical Research Centre (BRC), and Wellcome Leap Inc. announced that they had entered into an agreement to execute a Phase II clinical trial in patients with chronic obstructive pulmonary disease (COPD)-related sarcopenia.
Request a sample and discover the recent advances in sarcopenia drugs @ Sarcopenia Pipeline Report
The sarcopenia pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage sarcopenia drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the sarcopenia clinical trial landscape.
Sarcopenia Overview
Sarcopenia is a progressive condition associated with aging, marked by a decline in skeletal muscle mass, strength, and physical performance. It primarily affects the elderly and is linked to increased risks of falls, frailty, reduced mobility, and diminished quality of life. While age is the primary cause, other contributing factors include chronic illnesses, inflammation, hormonal changes, inadequate nutrition, and physical inactivity. Recognized by the World Health Organization (WHO) as a distinct disease, sarcopenia has become a growing focus in clinical research due to its significant impact on aging populations.
The underlying mechanisms of sarcopenia involve an imbalance in muscle protein turnover, mitochondrial dysfunction, deterioration of neuromuscular junctions, and elevated levels of inflammatory cytokines. Reduced levels of anabolic hormones like testosterone and growth hormone, combined with heightened catabolic activity, accelerate muscle breakdown. Insulin resistance and chronic low-grade inflammation also contribute to muscle loss and impaired regeneration, while a reduction in type II muscle fibers—essential for quick, powerful movements—worsens functional decline.
Sarcopenia is diagnosed based on assessments of muscle quantity, strength, and functional performance. Tools such as DXA (dual-energy X-ray absorptiometry) and BIA (bioelectrical impedance analysis) measure muscle mass, while handgrip strength and gait speed are commonly used to evaluate strength and mobility. Diagnostic frameworks like those from the European Working Group on Sarcopenia in Older People (EWGSOP) help standardize identification and classification, supporting more consistent clinical management.
Management and prevention primarily involve resistance training, adequate protein intake, and experimental pharmacological therapies. Strength training remains the gold standard for improving muscle function, while high-protein diets, particularly those rich in leucine, help stimulate muscle protein synthesis. Promising therapeutic options—such as myostatin inhibitors, selective androgen receptor modulators (SARMs), and anti-inflammatory treatments—are currently under investigation, though none are yet widely approved. As the global population continues to age, effectively addressing sarcopenia will be crucial for preserving independence and reducing age-related health burdens.
Find out more about sarcopenia drugs @ Sarcopenia Treatment
A snapshot of the Pipeline Sarcopenia Drugs mentioned in the report:
| Drugs | Company | Phase | MoA | RoA |
| BIO101 | Biophytis | II | Proto-oncogene protein c-mas-1 agonists | Oral |
| MF-300 | Epirium Bio | I | 15-hydroxyprostaglandin dehydrogenase (15-PGDH) enzyme inhibitor | Oral |
| BPM31510 | BPGbio, Inc. | I | Electron transport chain complex protein modulators; Proto-oncogene protein c-bcl-2 modulators | Oral |
| TRN005 | Turn Biotechnologies | Preclinical | RNA interference | NA |
| ES-1602 | EUSOL Biotech | Preclinical | Undefined mechanism | NA |
Learn more about the emerging sarcopenia therapies @ Sarcopenia Clinical Trials
Sarcopenia Therapeutics Assessment
The sarcopenia pipeline report proffers an integral view of the emerging sarcopenia therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Sarcopenia Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Oral, Intravenous, Subcutaneous, Parenteral, Topical
- Therapeutics Assessment By Molecule Type: Recombinant fusion proteins, Small molecule, Monoclonal antibody, Peptide, Polymer, Gene therapy
- Therapeutics Assessment By Mechanism of Action: Proto-oncogene protein c-mas-1 agonists, Hydroxyprostaglandin dehydrogenase inhibitors, RNA interference, Cell replacements, Electron transport chain complex protein modulators, Proto-oncogene protein c-bcl-2 modulators
- Key Sarcopenia Companies: Biophytis, TNF Pharmaceuticals, Epirium Bio, Turn Biotechnologies, Oncocross, EUSOL Biotech, BPGbio, Inc., Lipocine, and others.
- Key Sarcopenia Pipeline Therapies: BIO101, MYMD-1, MF-300, TRN005, ES-1602, OC514, BPM31510, and others.
Dive deep into rich insights for new sarcopenia treatments, visit @ Sarcopenia Drugs
Table of Contents
| 1. | Sarcopenia Pipeline Report Introduction |
| 2. | Sarcopenia Pipeline Report Executive Summary |
| 3. | Sarcopenia Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Sarcopenia Clinical Trial Therapeutics |
| 6. | Sarcopenia Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Sarcopenia Pipeline: Late-Stage Products (Phase III) |
| 8. | Sarcopenia Pipeline: Mid-Stage Products (Phase II) |
| 9. | Sarcopenia Pipeline: Early-Stage Products (Phase I) |
| 10. | Sarcopenia Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Sarcopenia Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Sarcopenia Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the sarcopenia pipeline therapeutics, reach out @ Sarcopenia Therapeutics
Related Reports
Sarcopenia Epidemiology Forecast
Sarcopenia Epidemiology Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted sarcopenia epidemiology in the 7MM, i.e., the United States, EU5 (Germany, Spain, Italy, France, and the United Kingdom), and Japan.
Sarcopenia Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key sarcopenia companies, including Biophytis, TNF Pharmaceuticals, Epirium Bio, Turn Biotechnologies, Oncocross, EUSOL Biotech, BPGbio, Inc., Lipocine, among others.
Osteoporosis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key osteoporosis companies, including Merck & Co. Inc., Amgen Inc., Radius Health Inc., Teva Pharmaceutical Industries Ltd, GlaxoSmithKline PLC, Pfizer Inc., Eli Lilly and Company, F. Hoffmann La Roche, Novartis International AG, Actavis PLC, Shanghai JMT-Bio Inc., Transcenta Holding, Celltrion, Sandoz, Enzene Bioscience, Fresenius Kabi, Gedeon Richter, Samsung Bioepis, Alvotech, MAbxience, Shanghai Henlius Biotech, among others.
Osteoporosis Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key osteoporosis companies, including mAbxience, Alvotech, Transcenta Holding, Suzhou Suncadia Biopharmaceuticals, GlycoNex, Cellatoz Therapeutics, Enzo Biochem, Surrozen, among others.
Osteoarthritis Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key osteoarthritis companies, including Paradigm Biopharma, Organogenesis, Amzell, Sorrento Therapeutics, Kolon TissueGene, Juniper Biologics, Biosplice Therapeutics, AKL Research and Development, BioSenic (Bone Therapeutics), Xalud Therapeutics, Eli Lilly and Company, Grünenthal, Techfields Pharma, Taiwan Liposome Company, UnicoCell Biomed, Medipost, Moebius Medical, Propella Therapeutics, Vizuri Health Sciences, Medivir, Novartis, BioSolution, Centrexion Therapeutics, Levicept, Merck KGaA, TrialSpark, Novo Nordisk, among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us
Shruti Thakur
info@delveinsight.com
+14699457679
www.delveinsight.com
Distribution channels: Banking, Finance & Investment Industry, Healthcare & Pharmaceuticals Industry, Media, Advertising & PR, Science ...
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release

