Global Pharmacovigilance Market Outlook, Size, Share & Trends Report, 2031
Pharmacovigilance Market to Reach USD 12.03 Billion by 2031 Driven by rise in research and development initiatives
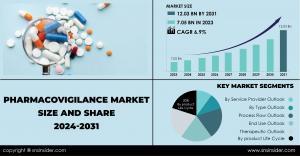
AUSTIN, TEXAS, UNITED STATES, June 21, 2024 /EINPresswire.com/ -- The SNS Insider report estimates the Pharmacovigilance Market Size at USD 7.05 billion in 2023, with a projected CAGR of 6.9% to reach USD 12.03 billion by 2031.Several factors are driving this market growth such as, the rise in research and development (R&D) initiatives, decentralized clinical trials, and new drug and vaccine launches all safety monitoring of these new products. key players are actively collaborating and launching innovative platforms to further strengthen the market. Also the increasing cases of chronic diseases like diabetes and cancer is leading to increased medication use, in which raises awareness about potential ADRs. For example, by implementing stricter reporting requirements for ADRs. The rising incidence of ADRs due to drug misuse and the growing need for combination of drug therapies, where patients take multiple medications simultaneously, are significant market drivers. Stringent government regulations, like those requiring comprehensive safety testing before drug approval, and the development of new drugs like personalized medicines and biosimilars are also contributing to the market's growth. As chronic diseases continue to rise, then global medication use is also rises, further increasing the demand for advanced pharmacovigilance services.
Drug safety concerns are due to a rise in adverse drug reactions (ADRs). For example, some medications used to treat chronic conditions like diabetes can lead to some side effects such as low blood sugar. This has created a strong demand for improved methods to track, analyze, and manage drug safety data. Regulatory bodies, like the US Food and Drug Administration (FDA), with a growing number of reported ADR cases. To address this challenge and ensure patient safety, healthcare organizations and pharmaceutical companies are investing in solutions that promote compliance with regulations like the FDA's Good Pharmacovigilance Practices, minimize risks from drug side effects, and ultimately improve patient well-being.
Download Free Sample Report of Pharmacovigilance Market @ https://www.snsinsider.com/sample-request/3095
List of Pharmacovigilance Companies Profiled in Report:
- Accenture
- ArisGlobal
- BioClinica Inc
- Capgemini
- ClinChoice (formerly FMD K&L)
- Clinquest Group B.V. (Linical Americas)
- Cognizant
- IBM Corporation
- ICON plc.
- IQVIA
- ITClinical
- Laboratory Corporation of America Holdings
- Parexel International Corp.
- TAKE Solutions Ltd.
- United BioSource LLC
- Wipro Ltd.
Key Market Segmentation
By product Life Cycle
• Pre-Clinical
• Phase I
• Phase II
• Phase III
• Phase IV
By Service Provider Outlook
• In-house
• Contract Outsourcing
By Type Outlook
• Spontaneous reporting
• Intensified ADR Reporting
• EHE mining
By Process Flow Outlook
• Case Data management
• Signal Detection
• Risk Management System
By Therapeutic Outlook
• Oncology
• Neurology
• Cardiology
• Respiratory systems
• Others
By End Use Outlook
• Pharmaceuticals
• Medical Device manufacturers
• Others
Segment Analysis
- By Product Life Cycle, phase IV dominated the segment in pharmacovigilance market with revenue share more than 73% in 2023, due to this is the final stage of testing (phase IV) which is key for finding side effects of drugs that weren't seen before.
- By Service Provider, contract outsourcing dominated segment of pharmacovigilance market with more than 69% market share in 2023 due to rising ADRs and CROs
- By Type, spontaneous reporting dominated the segment with more than 28% of market share in 2023 due to finding new side effect of drug faster and costeffective
- By Therapeutic Area, The oncology segment dominated the pharmacovigilance market with more than 25% market share in 2023 due to their powerful nature demands specialized monitoring and assessment to balance effectiveness with minimizing side effects.
Have Any Query? Ask Our Experts @ https://www.snsinsider.com/enquiry/3095
Regional Analysis
The North America dominated the pharmacovigilance market with more than 30% of market share. The presence of number of medical devices and pharmaceutical companies in North America is majorly contributes to the region's pharmacovigilance revenue. The growing problem of drug abuse and the resulting adverse drug reactions (ADRs) are driving the need of pharmacovigilance practices in the region. Increasing patient awareness and concerns regarding drug safety have impact on the North American pharmacovigilance market.
The Asia Pacific region also have growth in the pharmacovigilance market. This growth is due to Generic Drug Production, Heightened Focus on Drug Safety, Cost-Effective Clinical Trials
Recent Developments
- February 2024, Ergomed plc's PrimeVigilance, acquired Panacea. This acquisition strengthens PrimeVigilance's ability to offer a wider range of services in the areas of drug safety, regulatory affairs, quality assurance, biotech, and medical device industries.
- February 2023, Parexel International Corporation launched a new initiative name "Expert Series—New Medicines, Novel Insights." This program features fresh perspectives from Parexel's team of experts across various disciplines.
- December 2021, Wipro Limited announced the launch of regulatory literature monitoring, achieved through a new managed services agreement with Springer Nature. This partnership allows Wipro to offer a wider range of solutions to the pharmaceutical industry.
Key Takeaways
• The report clarifies the growing need for pharmacovigilance due to rising concerns about Adverse Drug Reactions (ADRs) and medication use for chronic diseases.
• The report emphasizes the dominance of Phase IV clinical trials (for monitoring side effects) and contract outsourcing (due to rising ADRs) in the Pharmacovigilance market.
• The report acknowledges the significant market share held by the oncology segment
• The report highlights North America's leading position in the market due to the presence of major pharmaceutical companies and growing awareness about drug safety.
Purchase Pharmacovigilance Market Report @ https://www.snsinsider.com/checkout/3095
Table of Content
Chapter 1 Introduction
Chapter 2 Research Methodology
Chapter 3 Pharmacovigilance Market Dynamics
Chapter 4 Impact Analysis (COVID-19, Ukraine- Russia war, Ongoing Recession on Major Economies)
Chapter 5 Value Chain Analysis
Chapter 6 Porter’s 5 forces model
Chapter 7 PEST Analysis
Chapter 8 Pharmacovigilance Market Segmentation, By Product Life Cycle
Chapter 9 Pharmacovigilance Market Segmentation, By Service Provider Outlook
Chapter 10 Pharmacovigilance Market Segmentation, By Type Outlook
Chapter 11 Pharmacovigilance Market Segmentation, By Process Flow Outlook
Chapter 12 Pharmacovigilance Market Segmentation, By Therapeutic Outlook
Chapter 13 Pharmacovigilance Market Segmentation, By End Use Outlook
Chapter 14 Regional Analysis
Chapter 15 Company profile
Chapter 16 Competitive Landscape
Chapter 17 Use Case and Best Practices
Chapter 18 Conclusion
Continued…
Other Trending Reports
Oncology Information System Market Outlook
Life Science Analytics Market Outlook
Akash Anand
SNS Insider Pvt. Ltd
+1 415-230-0044
email us here
Distribution channels: Healthcare & Pharmaceuticals Industry
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
Submit your press release