With COVID-19 patients across the country suffering due to a shortage of supplemental oxygen, the Drugs Controller General of India (DCGI) recently approved the emergency use of the drug 2-deoxy-D-glucose (2-DG) to treat moderate and severe cases of the SARS-CoV-2 infection. Apparently, 2-DG is said to reduce oxygen dependence and enable faster recovery.
The announcement was made in a press release not by any medical bodies or departments under the Indian government, but rather by the Ministry of Defence on 8 May 2021. It was developed by the Institute of Nuclear Medicine and Allied Sciences (INMAS) under the Defence Research and Development Organisation (DRDO) in collaboration with Hyderabad-based Dr Reddy's Laboratories (DRL).
2-DG 's therapeutic use for COVID-19 is set to begin in the coming week. It will be administered orally as a solution made from mixing a powdered 2-DG in water. However, what do we know about the 'immensely beneficial' drug? And is the approval for its application a statistically-informed decision? Let us take a look.
What is 2-DG?

2-DG is a derivative of glucose. It is an experimental drug and is said to function as an antiviral and anticancer agent. The drug is also a glycolytic inhibitor—suppresses the process of glycolysis, a metabolic pathway that is unregulated in cancer. 2-DG's use is not as the main treatment but as an adjunct therapy (i.e) it is administered along with primary treatment. Though it has been tested in cancer treatment, it has never been approved for the treatment of any diseases.
As a diagnostic agent, it is used in its radiolabelled (tagged with a radioactive substance) form. Through the utilization of positron emission tomography (PET), radiolabelled 2-DG helps in ascertaining changes in glucose metabolism, which is characteristic in diseases such as Alzheimer's disease, cancer and cardiovascular disease (CVD).
Its anticancer action is based on its property of being a glycolytic inhibitor. Glycolysis is the first step in the process of breakdown of glucose molecules for the purpose of extracting energy for cellular metabolism.
Cancer cells depend on anaerobic glycolysis for the purpose of producing energy in the form of Adenosine triphosphate (ATP). Being a modified form of glucose, 2-DG cannot undergo the process of glycolysis. Therefore, it can impede the survival of cancer cells. Its antiviral action is based on its ability to prevent glycosylation of particular lipids and proteins, and prevent the penetration of the virus into cells.
What Has Been Told?
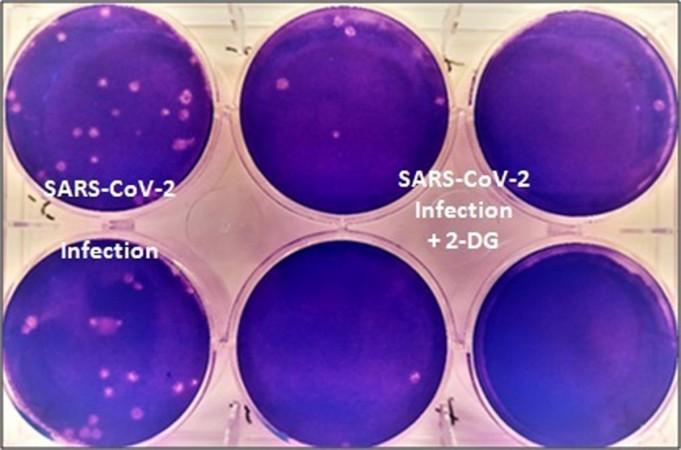
According to the press release, scientists from INMAS-DRO –with assistance from the Centre for Cellular and Molecular Biology (CCMB)—conducted laboratory experiments (in vitro) in which it was learnt that the 2-DG was effective against the coronavirus virus and prevented its growth. On the basis of these results, Phase-II clinical trials in COVID-19 patients were permitted by Central Drugs Standard Control Organization (CDSCO) in May 2020.
DRDO and DRL commenced clinical trials to investigate the efficacy and safety of 2-DG in COVID-19 patients. The safety of the drug was established in the Phase-II trials conducted between May to October 2020, and the patients are said to have shown improved recovery. Phase IIa was carried out in six hospitals. Phase IIb clinical trial, which involved dose-ranging, was conducted pan-India at 11 hospitals. 110 patients were part of the Phase-II trial.
"In efficacy trends, the patients treated with 2-DG showed faster symptomatic cure than Standard of Care (SoC) on various endpoints. A significantly favourable trend (2.5 days difference) was seen in terms of the median time to achieving normalisation of specific vital signs parameters when compared to SoC," the release stated.
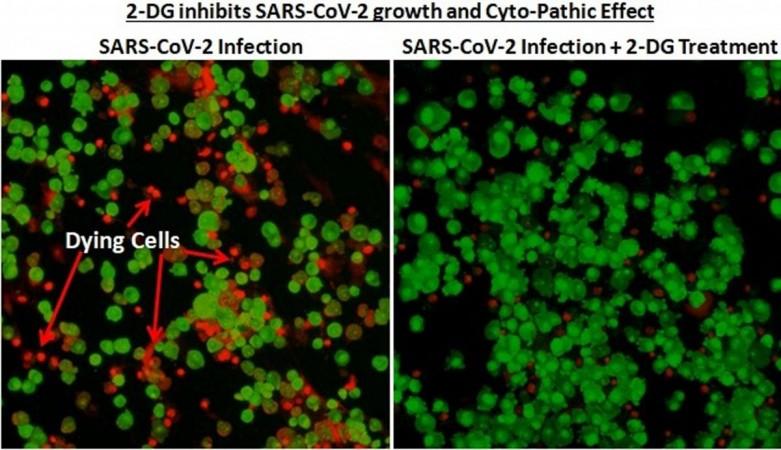
On the basis of these results, Phase-III clinical trials received DCGI's nod in November 2020. Conducted between December 2020 to March 2021, Phase-III clinical trial involved 220 patients from 27 COVID-19 hospitals across 10 states. "In 2-DG arm, significantly higher proportion of patients improved symptomatically and became free from supplemental oxygen dependence (42% vs 31%) by Day-3 in comparison to SoC, indicating an early relief from Oxygen therapy/dependence," declared the statement.
The release asserted further that similar trends were noted in patients who were above 65 years of age. Spurred by the claimed results, permission for emergency use of 2-DG as adjunct therapy patients with moderate to severe COVID-19 was granted by the DCGI on 1 May 2021.
Missing Crucial Information
While the drug is being touted as one that is "expected to save precious lives", there are no published studies about the trials from either DRDO or DRL. Therefore, there is no recorded data to validate the claimed results. No corresponding entries for the described Phase-II trials are available on the Clinical Trial Registry of India (CTRI) website. A registered Phase-II trial for 2-DG found in the registry consists of only 40 participants across 12 sites as against the 110 patients specified in the release.
According to the release, patients who were administered 2-DG went back to 'normal' 2.5 days earlier (on average) than those who had not received the drug. The criterion for normal, however, remains undefined. Also, normalization of vitals is part of the secondary endpoints or outcomes and not the primary in the CTRI entry. The release does not address the remainder of the primary and secondary outcomes and if they were all met.
Next, the release also utilizes terms such as "significantly higher" and "significantly favourable" to report results from the trials. Are these terms synonymous with 'statistically significant'? If that is the case, without statistical data from the trials, it is not possible to say if they are such. Additionally, the CTRI entry for Phase-III trials does not mention what primary outcomes/endpoints are and what the parameters for the evaluation are. Therefore, it is not possible to infer whether the observations mentioned in the release fall within the primary or secondary outcomes and if the others were met.
Though the release talks about the efficacy of the 2-DG in trials, no reference is made to its complete safety profile. With 110 and 220 participants said to have been enrolled in Phase-II and Phase-III clinical trials respectively, no mention of any observed side effects have been made. Therefore, the common to rare side-effects that soon to be treated patients may face remains unknown.












